Seeing memories form | ISTA scientists take a deep look into memory processing inside the hippocampus
Resembling a seahorse, as its name implies from the Greek words “hippos” (horse) and “kampus” (sea monster), the hippocampus is a brain region crucial for memory formation. But until recently, scientists have not been able to link memory formation to distinct molecular signals. Now, a team of scientists from the Institute of Science and Technology Austria (ISTA) and the Max Planck Institute for Multidisciplinary Sciences likely opened this black box. Their results are published in PLOS Biology.
Henry Gustav Molaison, known as patient ‘H.M.’, was suffering from epilepsy. Riddled by seizures, he was referred to a surgeon, who localized the epilepsy to the temporal lobe inside his brain, home of the hippocampus.
On September 1, 1953, H.M. underwent brain surgery, removing his hippocampus to cure his epilepsy. After the surgery, the epilepsy and seizures were gone, yet H.M. developed severe side effects. He was now suffering from anterograde amnesia, remembering all the events before the surgery but unable to form new memories. His case helped link the hippocampus to brain function and memory formation.
Nowadays, the hippocampus is recognized as a crucial region in the human brain, involved in memory formation and spatial navigation. It converts short-term memory into long-term memory, facilitating the revision of personal experience.
In a new study led by Olena Kim, Yuji Okamoto, and Magdalena Walz Professor for Life Sciences at the Institute of Science and Technology Austria (ISTA) Peter Jonas, an international team of neuroscientists uncovered new details about the molecular mechanisms that drive memory processing. The scientists took a precise look at the mossy fiber synapse—a key connection point between specific nerve cells (neurons) in the hippocampus—by combining approaches to study its structure, essential molecules, and functionality.
The memory center
Inside the hippocampus, several types of neurons are involved in memory processing. Granule cells, for instance, are important for handling incoming information. “Granule cells receive various signals from other brain regions, which they must process and propagate further,” explains Olena Kim, ISTA graduate and now a postdoc at the Institute of Molecular Biotechnology (IMBA) of the Austrian Academy of Sciences (ÖAW).
These signals get transmitted through the granule cells’ axons—their arm-like extension, known as mossy fibers. These fibers form a contact point to pyramidal cells—the mossy fiber synapse. At this connection, messenger molecules in the form of neurotransmitters facilitate communication, eventually triggering the formation and storage of memory down the line.
Mossy fiber synapses are characterized by their high plasticity, meaning they can change their activity, structure, and connections based on stimuli. This adaptability helps the hippocampus to process information correctly and distinguish between similar cues.
Kim gives an example, “Let's assume, you encounter a panther and a black cat simultaneously, both appear black and feline. Yet you can distinguish one as a cat and one as a panther. Mossy fiber synapses play a key role in encoding and processing these distinguishing features, eventually retrieving memory and information.”
Mossy fiber synapses in close-up
The exact molecular details of how signal processing in the mossy fiber synapses works are still unknown. In 2020, Peter Jonas, Carolina Borges-Merjane, and Olena Kim set out to study the structure of mossy fiber synapses, by using a new technique called ‘Flash and Freeze’—a powerful tool, where neurons are frozen right after being stimulated.
“Back then, we were able to correlate structural changes in the mossy fiber synapses to their functionality,” says Kim. “However, we wanted to push the technique further and not only look at the structure of the synapses, but also at the changes that occur on the molecular level when signals get processed.”
The scientists were particularly interested in two proteins located at the neurotransmitter release zone: Cav2.1 calcium channels, which are crucial as the influx of calcium through those channels triggers neurotransmitter release, and Munc13, a key factor, that hints at the neurotransmitter’s readiness to be released.
“Before our study, all the work on these two proteins was done with chemically fixed brain samples,” Kim continues. As those samples are not alive, they do not provide insights into dynamic processes. “For our new study, we were eager to use live brain tissue to preserve the dynamics, the natural compositions, and the localization of these proteins.”
A moon-like surface
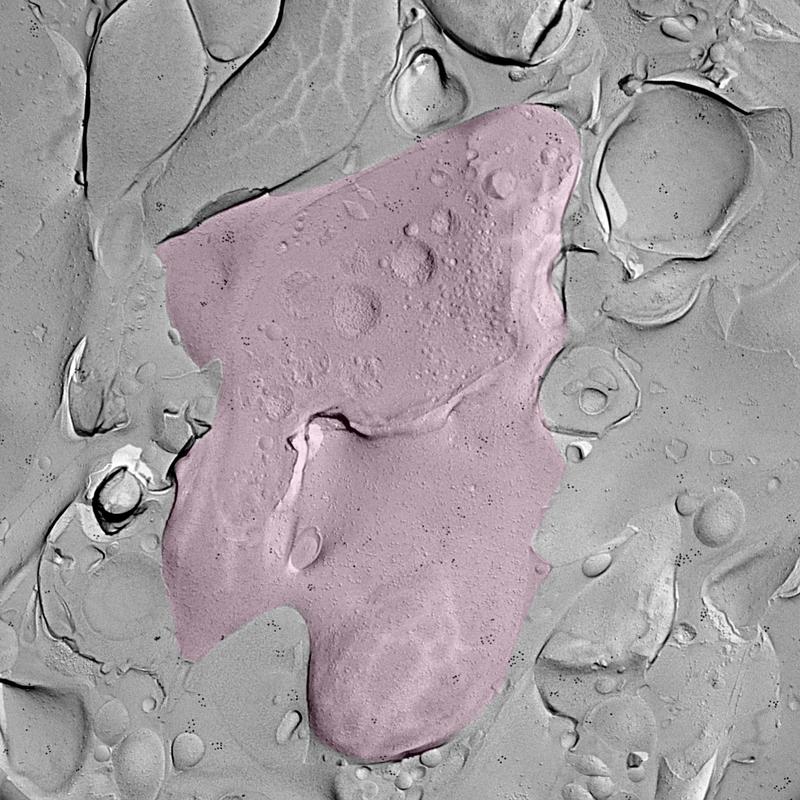
With the help of their ISTA colleagues, Professor Ryuichi Shigemoto, and Staff Scientist Walter Kaufmann, the scientists used the ‘freeze fracture labeling’ technique. They chemically stimulated the granule cells in mouse brain tissue samples to activate the process of memory formation. Then, the brain tissue was instantly frozen and split apart into two halves. The inner side of the section represents the exposed surface of the tissue inside—a 3D footprint of the tissue in that specific moment, with embedded proteins and molecules.
After labeling Cav2.1 and Munc13 to make them visible, the researchers used an electron microscope to find their exact localization. The images, resembling a close-up of the moon, revealed that upon stimulation, these two proteins rearranged and moved closer together.
Further testing revealed that the rearrangement is tightly correlated with the functionality of the mossy fiber synapse. Peter Jonas summarizes, “Upon activation, there are two changes. First, the number of vesicles near the membrane increases. Second, there is nano-rearrangement of Cav2.1 and Munc13, making the synapses more powerful and more precise. Both changes may contribute to memory formation.”
The study sheds light on the relationship between structure and function at a key synapse in the hippocampus. Our memories often evoke vivid images. But until now, we have not been able to capture the molecular signals that unleash memory formation. The present study sets a cornerstone in that.
-
Funding information
This project was supported by funding from the European Research Council and the European Union’s Horizon 2020 research and innovation program (ERC 692692 to P.J.) and from the Fond zur Förderung der Wissenschaftlichen Forschung (Z312-B27 697 Wittgenstein award to P.J.; W1205-B09 and P36232-B to P.J.; I6166-B to R.S.).
Information on animal studies
In order to better understand fundamental processes, for example, in the fields of neuroscience, immunology, or genetics, the use of animals in research is indispensable. No other methods, such as in silico models, can serve as alternative. The animals are raised, kept, and treated according to the strict regulations of Austrian law. All animal procedures are approved by the Federal Ministry of Education, Science and Research.
Originalpublikation:
O. Kim, Y. Okamoto, W. A. Kaufmann, N. Brose, R. Shigemoto & P. Jonas. 2024. Presynaptic cAMP-PKA-mediated potentiation induces reconfiguration of synaptic vesicle pools and channel-vesicle coupling at hippocampal mossy fiber boutons. PLOS Biology. DOI: 10.1371/journal.pbio.3002879
https://journals.plos.org/plosbiology/article?id=10.1371/journal.pbio.3002879
Weitere Informationen:
https://ista.ac.at/en/research/jonas-group/
https://ista.ac.at/en/research/shigemoto-group/
Die semantisch ähnlichsten Pressemitteilungen im idw