Personalized medicine - Functioning, induced liver cells from skin tissue
A research group from Inselspital, Bern University Hospital, Children's Hospital Zurich - Eleonore Foundation and the University of California San Francisco (UCSF) has succeeded in taking an important step towards generating personalized, artificial liver cells. For the first time, stem cells generated from skin tissue could be made to function like normal liver cells by adding a transport protein. These cells can now be used to test therapeutic agents, among other things.
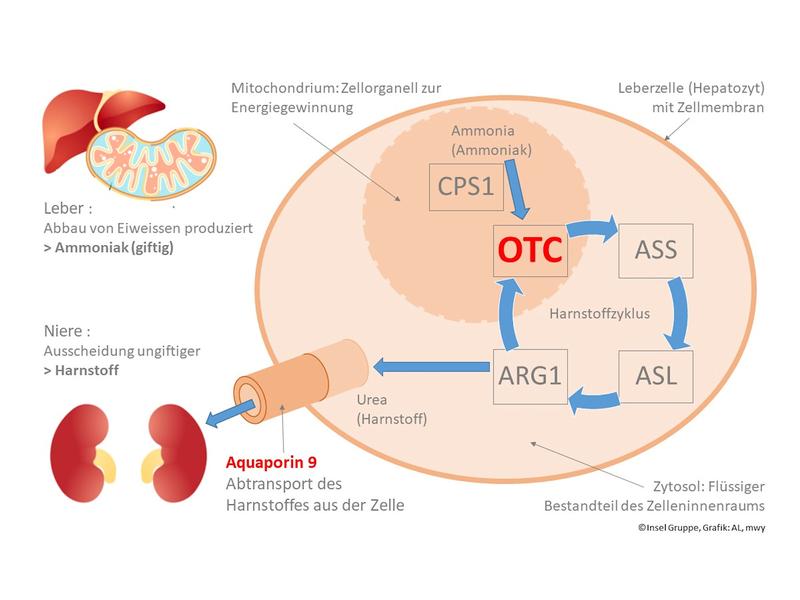
The urea cycle is responsible for removing nitrogenous waste produced from the breakdown of protein in the organism. If the OTC enzyme is missing in this cycle, ammonia accumulation rises to toxic levels. An OTC deficiency is the most common disorder in the urea cycle. It could not be cured with drugs until now.
The OTC gene is located on the sex chromosome (X chromosome). This means that the manifestation of the disorder is usually weaker in female newborns. However, in male newborns, who have one X and one Y chromosome, a deficiency in the OTC gene has a dramatic effect: In newborn boys, ammonia toxicity due to OTC deficiency is often fatal. The research team looked for ways to test drugs in the lab against the OTC deficiency.
Created an initial model
First the research team generated liver cells from patients’ skin tissue in an elaborate process. It worked like this: Initially, a tissue sample of the skin was taken from OTC-deficient patients as well as from a control group (healthy individuals). In a complex process, the samples were differentiated so that they functioned like stem cells. This engineering process was developed by Shin’Ya Yamanaka, for which he was awarded the Nobel Prize in Medicine in 2012. “By using induced stem cell technology, we succeeded in generating liver cells that function largely like liver cells from patients,” explains Dr. med. Alexander Lämmle, Senior Physician at the Department of Pediatrics and the University Institute of Clinical Chemistry at Inselspital. “However, we observed that the induced liver cells excrete significantly less urea than real, healthy liver cells, and this is independent of whether they originate from healthy controls or urea cycle patients.” The researchers were able to determine the reason for this behavior. The technologically engineered stem cells were characterized by a complete lack of aquaporin 9, a transport protein in the cell membrane. The reason for this deficiency is the still immature, fetal character of the artificial liver cells.
Aquaporin 9: the key to a functioning artificial liver cell,
Aquaporins organize the transport of water and certain substances through the cell membrane. Aquaporin 9 is responsible for the transport of urea. In a next step, the researchers developed a process in which the formation of aquaporin 9 is induced in the stem cells. As a result, the technologically produced liver cells changed their behavior. They were able to break down ammonia into urea and excrete the urea – just as healthy cells do. This provides the basis for a functioning test procedure with artificial liver cells.
Use of artificial cells: testing therapeutic agents
The OTC deficiency is characterized by the fact that the complex OTC protein structures do not function properly. They need –¬ like most larger proteins –¬ helpers or chaperones in order to assemble and function properly. Prof. Dr. med. Johannes Häberle at the Children Research Center at the University Hospital Zurich explains: “The chaperones ensure that the enzyme molecules are folded correctly and that the enzyme is correctly prepared for its use or reset afterwards. The new test model is now being used to test OTC chaperones to find out more about the OTC deficiency and, of course, about possible therapies.”
Wissenschaftlicher Ansprechpartner:
- Dr. med. Alexander Laemmle, Department of Pediatrics, Inselspital, Bern University Hospital and University Institute of Clinical Chemistry, University of Bern.
- Prof. Dr. med. Johannes Häberle, Senior Attending Physician, Head of Metabolism Laboratory, Zurich Center for Integrative Human Physiology (ZIHP), University of Zurich.
- Prof. Holger Willenbring, M.D., Ph.D., Professor of Surgery, Eli and Edythe Broad Center of Regeneration Medicine and Stem Cell Research, Associate Director, Liver Center, University of California, San Francisco
Originalpublikation:
Laemmle, A., Poms, M., Hsu, B., Borsuk et al. (2021): Aquaporin 9 Induction in Human iPSC-derived Hepatocytes Facilitates Modeling of Ornithine Transcarbamylase Deficiency. Hepatology. Accepted Author Manuscript
- DOI: https://doi.org/10.1002/hep.32247
Ähnliche Pressemitteilungen im idw