How are inflammation, aging, and diet related? The systemic regulatory network described for the first time
Mild, persistent inflammation in tissue is considered one of the biological hallmarks of the aging process in humans – and at the same time is a risk factor for diseases such as Alzheimer’s or cancer. Prof. Francesco Neri and Dr. Mahdi Rasa of the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) in Jena have succeeded for the first time in describing at the molecular level the regulatory network that drives the general, multiple-organ inflammatory response. Moreover, they were able to show that dietary restriction can influence this regulatory circuit, thereby inhibiting inflammation.
Jena. Inflammation is an immune response of the body that is, in itself, useful: our immune system uses it to fight pathogens or to remove damaged cells from tissue. Once the immune cells have done their work, the inflammation subsides: the infection is over, the wound is healed. Unlike such acute inflammations, age-related chronic inflammation is not local. The innate immune system ramps up its activity overall, resulting in chronic low-grade inflammation. This aging-related inflammation is also known as inflammaging.
Inflammaging impairs health
This underlying inflammation has consequences for health: “If you have a constant activation of immune cells, this can lead to their exhaustion, which in turn leads to problems when you have an infection. Immune cells may no longer respond properly. Inflammaging is also related to cancer development – because in inflamed tissue we see increased cell proliferation,” explains Prof. Francesco Neri, who headed the research group “Epigenetics of Aging” at the Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) in Jena until the end of 2021. The biologist is now teaching and conducting research at the University of Turin, Italy.
How do aging, inflammation, and diet interact?
Together with Dr. Mahdi Rasa and other colleagues at the FLI, Prof. Neri conducted a study in mice to investigate how the chronic inflammation that accompanies aging is regulated and maintained by genes – and whether dietary restriction can influence this regulatory network and inhibit inflammation. Indeed, studies over the past two decades have shown that various animals – from flies to worms to rodents to monkeys – live longer when fed a diet that reduces caloric intake. For example, when mice were fed 30 percent less food, they were fitter, more active, and lived three to four months longer – the equivalent of a 10 to 15 percent increase in life span. Improved health has also been observed in humans on calorie-restricted diets. It is also known that inflammatory responses can be reduced by this dietary approach. However, the ways in which inflammation, aging, and dietary restriction are regulated in detail at the molecular level, as well as how they relate to each other, are not yet understood.
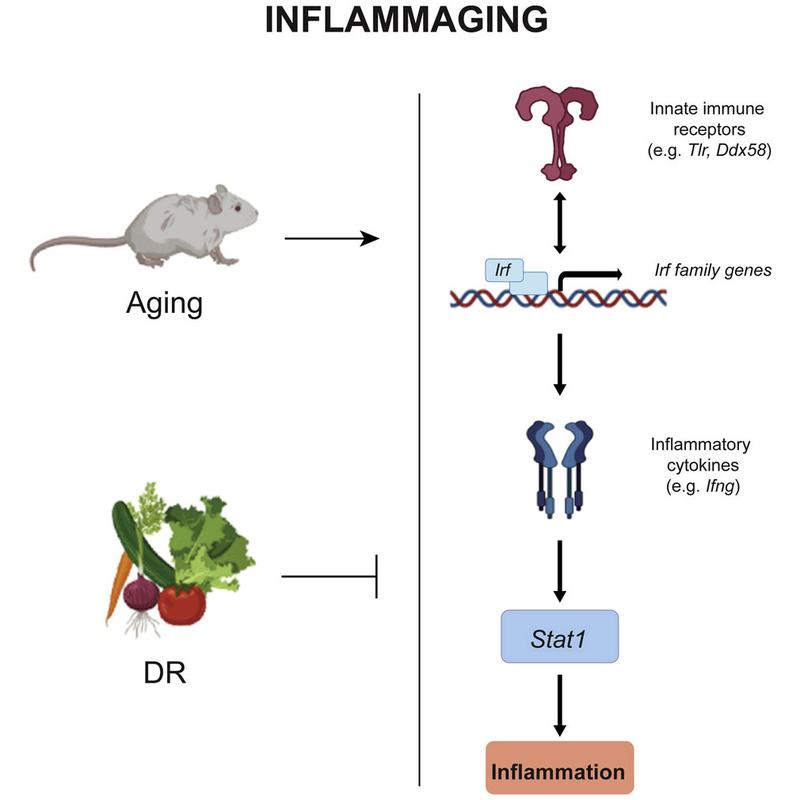
Feedback loop of inflammation
For their current study, which was recently published in the journal Cell Reports, the FLI researchers first compared four-month-old mice with older mice (22 months). In contrast to previous studies, the activity of the genes was measured not only in one organ, but in multiple tissues in parallel: blood, brain, heart, kidney, liver, lung, muscle, and skin. “The priority was to study the pathways that were affected in all tissues in order to understand inflammaging on a systemic level,” explains Neri. “What we found is that the inflammatory stage in older mice was characterized by the upregulation of a specific set of genes that encodes receptors of the innate immune system. This upregulation leads to the activation of a set of interferon-regulating genes. And these genes then activate other genes that produce inflammatory cytokines, as well as activating Stat1, which is a master transcription factor for the regulation of genes associated with inflammation. This entire process is like a positive feedback loop that keeps the inflammatory state going.” The Jena researchers are thus the first to describe the cross-organ and thus systemic regulatory network (see picture below). But can this cycle be interrupted by a reduced calorie intake?
Long and short-term dietary restriction shows positive effects
In order to answer this question, Neri and Rasa studied the gene activity in the organs of two additional groups of rodents: mice that were given 30 percent less food for almost their entire lives (4 to 22 months) and mice that were kept under these conditions for only two months at the end of their lives. Whether the caloric restrictions were short- or long-term, overall the diet had a positive effect on all organs studied, with the exception of the heart.
Systemic regulatory network is starting point for interventions
With their work, the two researchers also provide starting points for future drug therapies for aging-related chronic inflammation. “Within this regulatory network we describe, one well-studied and important component is, for example, TLR4, a gene that encodes a receptor of the innate immune system,” explains Dr. Rasa, who performed the gene analyses as part of his PhD thesis. “The receptor acts like an SOS signal that we don’t need when there are no pathogens to fight. If we could downregulate TLR4, we would be able to reduce the chronic inflammatory response in aging.”
Another much-discussed possibility for intervention is the administration of dietary supplements such as vitamins or probiotics with the aim of influencing the composition of microorganisms in the digestive tract. “Dietary restriction appears to change the microbiome, which leads to reduced inflammaging. If it is possible to change the intestinal flora of the microbiome through dietary supplements, the same beneficial effects could be achieved without the need for a restricted diet.” This is still only speculation, Prof. Neri admits. “We first need a better understanding of the processes involved.”
Contact
Kristina Vaillant
Communications Manager
Phone: +49 (0)3641-65-6373
Email: presse@leibniz-fli.de
----------------------------------------------------------------------------------------------------------------
Background information
The Leibniz Institute on Aging – Fritz Lipmann Institute (FLI) – upon its inauguration in 2004 – was the first German research organization dedicated to research on the process of aging. More than 350 employees from around 40 nations explore the molecular mechanisms underlying aging processes and age-associated diseases. For more information, please visit www.leibniz-fli.de.
The Leibniz Association connects 97 independent research institutions that range in focus from natural, engineering and environmental sciences to economics, spatial and social sciences and the humanities. Leibniz Institutes address issues of social, economic and ecological relevance. They conduct basic and applied research, including in the interdisciplinary Leibniz Research Alliances, maintain scientific infrastructure, and provide research-based services. The Leibniz Association identifies focus areas for knowledge transfer, particularly with the Leibniz research museums. It advises and informs policymakers, science, industry and the general public. Leibniz institutions collaborate intensively with universities – including in the form of Leibniz ScienceCampi – as well as with industry and other partners at home and abroad. They are subject to a transparent, independent evaluation procedure. Because of their importance for the country as a whole, the Leibniz Association Institutes are funded jointly by Germany’s central and regional governments. The Leibniz Institutes employ around 20,500 people, including 11,500 researchers. The financial volume amounts to 2 billion euros. For more information: www.leibniz-gemeinschaft.de/en/.
Originalpublikation:
Inflammaging is driven by upregulation of innate immune receptors and systemic interferon signaling and is ameliorated by dietary restriction. Seyed Mohammad Mahdi Rasa, Francesco Annunziata, Anna Krepelova, Suneetha Nunna, Omid Omrani, Nadja Gebert, Lisa Adam, Sandra Käppel, Sven Höhn, Giacomo Donati, Tomasz Piotr Jurkowski, Karl Lenhard Rudolph, Alessandro Ori, Francesco Neri. Cell Reports 2022, 39 (13) doi.org/10.1016/j.celrep.2022.111017
Ähnliche Pressemitteilungen im idw