Neutralising antibodies that target resistant bacteria
Antibiotic-resistant bacteria pose a major challenge to healthcare systems worldwide. Due to numerous resistance mechanisms, infections with the pathogen Pseudomonas aeruginosa are particularly feared. Researchers at the German Center for Infection Research (DZIF), the University Hospital Cologne, the Helmholtz Centre for Infection Research in Braunschweig and the University Medical Center Hamburg-Eppendorf have now discovered antibodies that could lead to a highly potent treatment option of acute and chronic infections with P. aeruginosa. The study was published in the prestigious scientific journal Cell.
Antibiotic-resistant bacteria pose a growing threat worldwide, not only to infected patients but also to our health systems as a whole. In particular, infections with the bacterium Pseudomonas aeruginosa are feared due to numerous resistance mechanisms and can lead to complicated lung and bloodstream infections, especially in seriously ill patients. In addition, the pathogen can permanently colonise organs such as the lungs, where it promotes progressive tissue damage. Often, so-called antibiotics of last resort have to be used in infected patients because the standard therapies are no longer effective. New therapeutic approaches are therefore urgently needed to ensure effective treatment of infections with multidrug-resistant pathogens such as P. aeruginosa in the future.
Researchers from the German Center for Infection Research (DZIF) and the University Hospital Cologne, together with colleagues from the Helmholtz Centre for Infection Research in Braunschweig and the University Medical Center Hamburg-Eppendorf, have now succeeded in isolating and characterising highly effective antibodies against this pathogen from immune cells of cystic fibrosis patients who were chronically infected with P. aeruginosa.
Research approach adopted from the development of antiviral therapies
"Many of the highly effective and broadly neutralising monoclonal antibodies used against viruses were isolated from infected, recovered or vaccinated individuals and then further developed for clinical use," explains Dr Alexander Simonis, first author of the study, assistant physician in the Department of Infectiology and head of the junior research group “Immunotherapies against bacterial infections” at the University Hospital Cologne.
In their study, the researchers therefore investigated whether the approach of isolating and recombinantly expressing broadly neutralising antibodies from human immune cells, which has been successful for viral infections, can also be used to develop new therapies against bacterial infections. In order to find suitable antibodies, they focused on patients with cystic fibrosis, whose lungs are often chronically colonised with P. aeruginosa. The researchers hypothesised that repeated exposure to the bacterium in these patients would lead to the development of antibodies able to effectively inhibit the virulence of P. aeruginosa. Thanks to a robust screening test, the researchers indeed found monoclonal antibodies in the blood samples of some cystic fibrosis patients that are able to neutralise the virulence of the bacterium.
Antibodies as pathoblockers: successful inhibition of bacterial virulence
The mode of action of these antibodies is based on blocking an important virulence factor of the bacterium, the so-called type III secretion system, which plays an important role especially in severe infections with P. aeruginosa. In extensive experiments with cell culture and animal models, the researchers were able to show that the newly developed antibodies are just as effective against the bacterium as classical antibiotics. However, since the activity of these antibodies is independent of the mechanisms of action of conventional antibiotics, these so-called pathoblockers can also—in contrast to many classical antibiotics—be effective against highly resistant bacteria.
"The gained knowledge and the applied experimental methods can also be transferred to other bacterial pathogens and thus represent a promising new approach to the therapy of infections with multidrug-resistant bacteria," summarises the study's lead author and DZIF scientist Dr Jan Rybniker, senior attending physician in the Department of Infectiology at the Clinic I for Internal Medicine and head of the "Translational Research Unit – Infectious Diseases" at the University Hospital Cologne. "We would also like to thank in particular all the patients who provided their blood for our investigations and thus contributed to our success," adds Rybniker.
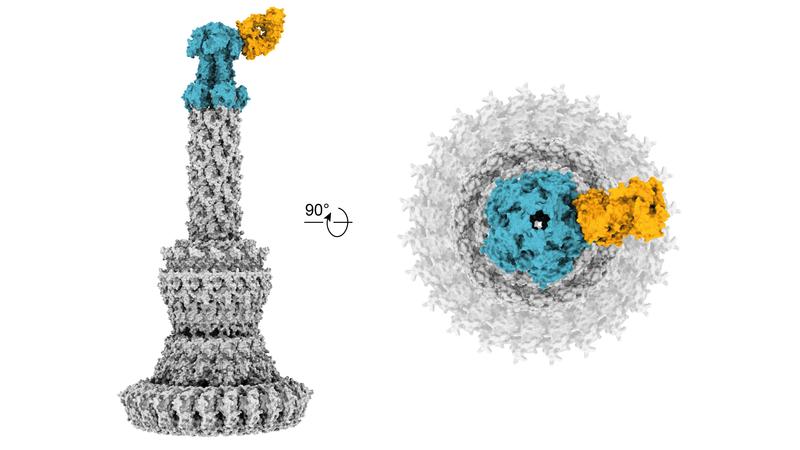
The study is based on close cooperation between scientists from several German research institutions. Relevant for the successful development of the antibodies was the cooperation with other researchers at the University Hospital Cologne. These included in particular Dr Christoph Kreer and Prof. Florian Klein, DZIF scientist and head of the Institute of Virology at the University Hospital Cologne, who contributed their extensive expertise in the field of antibody isolation against viral pathogens such as HIV-1 and SARS-CoV-2. A collaboration with Dr Ernst Rietschel and Dr Silke van Koningsbruggen-Rietschel, head of the Cystic Fibrosis Centre Cologne at the University Children´s Hospital, was pivotal for recruiting patients. Dr Katharina Rox, head of the DZIF Pharmacokinetics/Pharmacodynamics unit at the Helmholtz Centre for Infection Research in Braunschweig, was able to show that the new antibodies have very good efficacy in animal models. At the CSSB Centre for Structural Systems Biology in Hamburg—a cooperation with the University Medical Center Hamburg-Eppendorf—Dr Biao Yuan and Prof. Thomas Marlovits used cryo-electron microscopy and single particle analysis to decipher the exact structure and atomic details of the binding epitopes of the new antibodies for the Pseudomonas-specific target molecule.
The scientists are now planning to further refine the antibodies developed in the study together with the DZIF and other research supporters and to test them in clinical trials. In the long term, the antibodies are to be used as a new therapeutic approach, especially for acute and severe infections with P. aeruginosa. According to the researchers, the antibodies also offer the possibility of protecting patients with an increased risk of P. aeruginosa infections—especially in intensive care units or in cancer patients—by means of passive immunisation.
Wissenschaftlicher Ansprechpartner:
Dr Jan Rybniker
University Hospital Cologne
jan.rybniker@uk-koeln.de
Dr Alexander Simonis
University Hospital Cologne
alexander.simonis@uk-koeln.de
Originalpublikation:
Simonis A. et al., Discovery of highly neutralizing human antibodies targeting Pseudomonas aeruginosa, Cell, Nov 2023, https://doi.org/10.1016/j.cell.2023.10.002
Weitere Informationen:
https://www.dzif.de/en/neutralising-antibodies-target-resistant-bacteria DZIF press release
https://innere1.uk-koeln.de/forschung/arbeitsgruppen-labore/translational-research-unit-infectious-diseases/translational-research-unit-infectious-diseases-en/ translational research unit – infectious diseases
Ähnliche Pressemitteilungen im idw