Don’t overeat: How archaea toggle the nitrogen-uptake switch
By tightly regulating nitrogen uptake, microorganisms avoid overeating nitrogen and thus wasting energy. Scientists from the Max Planck Institute for Marine Microbiology now reveal how some methanogenic archaea manage to do so.
Life is not possible without nitrogen. There are many ways for organisms to acquire nitrogen. For example, humans eat proteins for their high nitrogen content. Most microorganisms take up nitrogen from their environment in the form of ammonia (NH3). As this process consumes cellular energy – which is usually scarce –, it needs to be tightly regulated. A team of scientists around Tristan Wagner from the Max Planck Institute for Marine Microbiology in Bremen, Germany, has now investigated this regulation in an underexplored group of microorganisms: the archaea.
Archaea and bacteria might be generally similar in size and shape but belong to two different kingdoms. The regulation of nitrogen uptake in bacteria is well-studied and very complex. In comparison, little is known about the process in archaea. “Thus, we set out to investigate how these underexplored microbes manage that regulation,” says first author Marie-Caroline Müller. Together with scientists from Radboud University in the Netherlands, they investigated the nitrogen-assimilating enzyme from two methane-generating archaea. Their results are now published in the journal Communications Biology.
One molecular switch, two ways to switch it
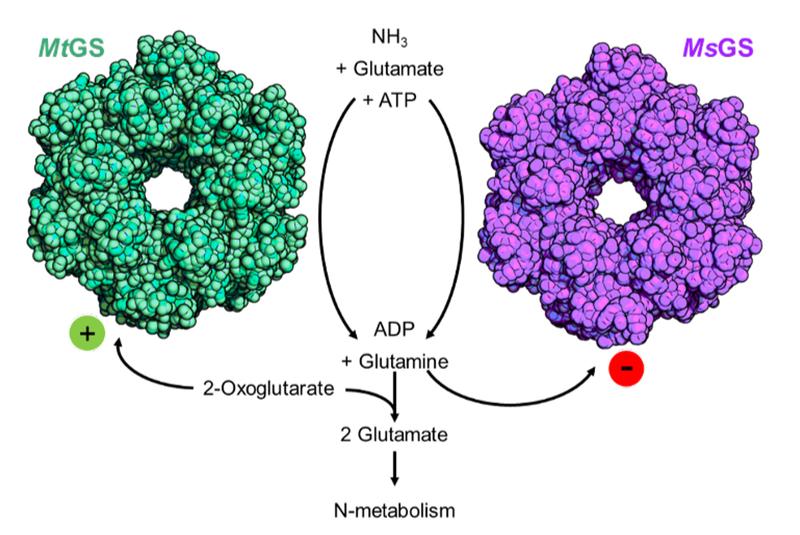
In their experiments, the scientists used two methane-producing archaea from very different environments: Methanothermococcus thermolithotrophicus comes from sediments in the Gulf of Naples, and Methermicoccus shengliensis, stems from an oilfield in China.
“While the studied organisms are generally quite similar, we found that they are remarkably different when it comes to regulating nitrogen uptake”, Müller explains. They rely on the same enzyme to obtain their nitrogen but operate the on/off switch differently.
First, the researchers purified the glutamine synthetase – the enzyme responsible for the energy-dependent uptake of nitrogen – from both organisms and checked its activity. There, they met their first surprise: While the enzyme from M. shengliensis was active as expected, the one from M. thermolithotrophicus was not. Obviously, both organisms used the same enzyme for nitrogen uptake but regulated it differently – i.e. they had different ways to turn it on and off. What was the trigger? The researchers decided to try out 2-oxoglutarate, which has been shown to boost the activity of glutamine synthetase in some archaea. And indeed, it worked. “I was very excited when I saw that result”, says Müller. “We had found a way to turn on the switch for the enzyme’s activity in M. thermolithotrophicus, and we even managed to regulate it by manipulating the concentration of 2-oxoglutarate: When the cell needs nitrogen, the 2-oxoglutarate levels increase and switch on the glutamine synthetase. However, when the cell has enough nitrogen, the 2-oxoglutarate levels decrease to a concentration that is insufficient to maintain the enzyme active, preventing nitrogen assimilation. Thus, no energy is wasted when the cell is well-supplied with nitrogen.”
Additionally, the scientists found that the M. shengliensis enzyme is inactivated by glutamine, the molecule carrying the nitrogen in the cell. This strategy has already been found in some bacteria. In contrast, M. thermolithotrophicus is insensitive to glutamine, illustrating once more a very different type of regulation between both microbes.
Close microbes, different mechanisms
Next, the scientists wanted to find out how the 2-oxoglutarate manages to turn on the nitrogen uptake switch in M. thermolithotrophicus. By X-ray crystallography, using synchrotron radiation, they determined the 3D structure of the enzyme, which had to be crystallized beforehand, with and without 2-oxoglutarate attached to it. Moreover, they compared it with the other investigated organism, M. shengliensis. In M. thermolithotrophicus, the 2-oxoglutarate binds in a pocket far away from the active site where the reaction takes place. The 2-oxoglutarate binding remodeled the enzyme to adopt the active form. However, the 2-oxoglutarate cannot bind to M. shengliensis´ enzyme, which explains why it is not reacting to 2-oxoglutarate. “Now we can describe in great detail how the 2-oxoglutarate switch works and which enzymes would be sensitive to it”, Müller says.
“Even close microbes, such as the one archaea generating methane, have engineered different ways to regulate nitrogen assimilation”, Wagner sums up. “There is a beauty in this regulatory network that has been polished by evolution to specifically and rapidly answer the needs of the cell. Further surprises can be expected: Most likely, other unexpected regulatory systems are waiting to be discovered”, Wagner concludes.
Wissenschaftlicher Ansprechpartner:
Marie-Caroline Müller
Research Group Microbial Metabolism
Max Planck Institute for Marine Microbiology, Bremen, Germany
Phone: +49 421 2028-9740
E-Mail: mmueller@mpi-bremen.de
Dr. Tristan Wagner
Head of the Research Group Microbial Metabolism
Max Planck Institute for Marine Microbiology, Bremen, Germany
Phone: +49 421 2028-7440
E-Mail: twagner@mpi-bremen.de
Originalpublikation:
Marie-Caroline Müller, Olivier N. Lemaire, Julia M. Kurth, Cornelia U. Welte, Tristan Wagner (2024): Differences in regulation mechanisms of glutamine synthetases from methanogenic archaea unveiled by structural investigations. Comm. Biol. 2024, publication date January 19, 2024.
DOI: 10.1038/s42003-023-05726-w
Weitere Informationen:
https://www.mpi-bremen.de/en/Page6155.html
Die semantisch ähnlichsten Pressemitteilungen im idw