The laborious path of a fungal toxin
New insights into the release of Candidalysin promise progress in the treatment of Candida albicans infections
The toxin Candidalysin of the yeast Candida albicans is incorporated into an unusual protein structure during an infection, the composition of which has so far been unknown to scientists. Researchers at the Leibniz-HKI have now succeeded in deciphering the function of this unusual arrangement. By modifying the protein structure, the pathogenicity of the fungus could also be reduced. The new findings were used to render the fungal toxin harmless with the help of artificial antibodies. This opens up a new way of treating persistent forms of vaginal Candida infection.
An opportunistic fungus
The yeast Candida albicans is part of the human microbiome and normally lives in balance with other microorganisms. However, if this balance is disturbed, the fungus can grow uncontrollably and cause infections. As a “vaginal thrush”, C. albicans affects millions of women worldwide every year. In some cases, antifungal treatment fails and chronic, recurring fungal infections occur, which significantly reduce the quality of life of those affected and cause a great deal of suffering.
In a wrinkled garment
The toxin Candidalysin secreted by the fungus is responsible for cell damage in human tissue. A vaginal infection typically results in severe inflammatory reactions, but these are of little concern to the fungus. Instead, the severity of the disease increases.
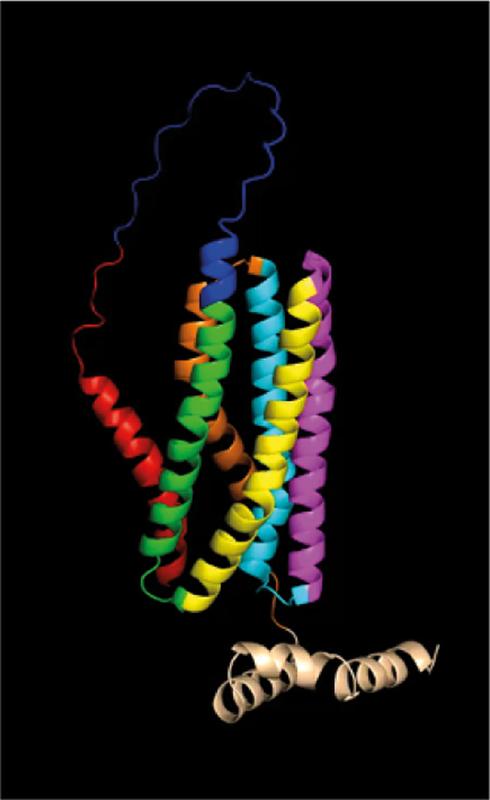
Candidalysin is embedded in a precursor protein called Ece1 before release: A total of eight peptide sequences form an unfolded structure, which is very unusual for such toxins. It was previously unclear why the toxin is integrated into this unusual structure and what role the individual components play. In a study just published in Nature Microbiology, an international team of researchers from the Leibniz-HKI, the Institut Pasteur Paris, the King’s College London, the University of Wisconsin and the Leibniz Research Center Borstel therefore investigated the structure of Ece1 and certain peptides from it, known as NCEPs (non-candidalysin Ece1 peptides).
With almost all microbial peptide toxins, the producing cells must take precautions against self-poisoning. Free Candidalysin is an exception here, as it does not damage its own fungal cells. Accordingly, its embedding in the complex precursor protein Ece1 cannot be explained by self-protection of the fungal cell. The reason for the unusual structure of Ece1 must therefore be something else.
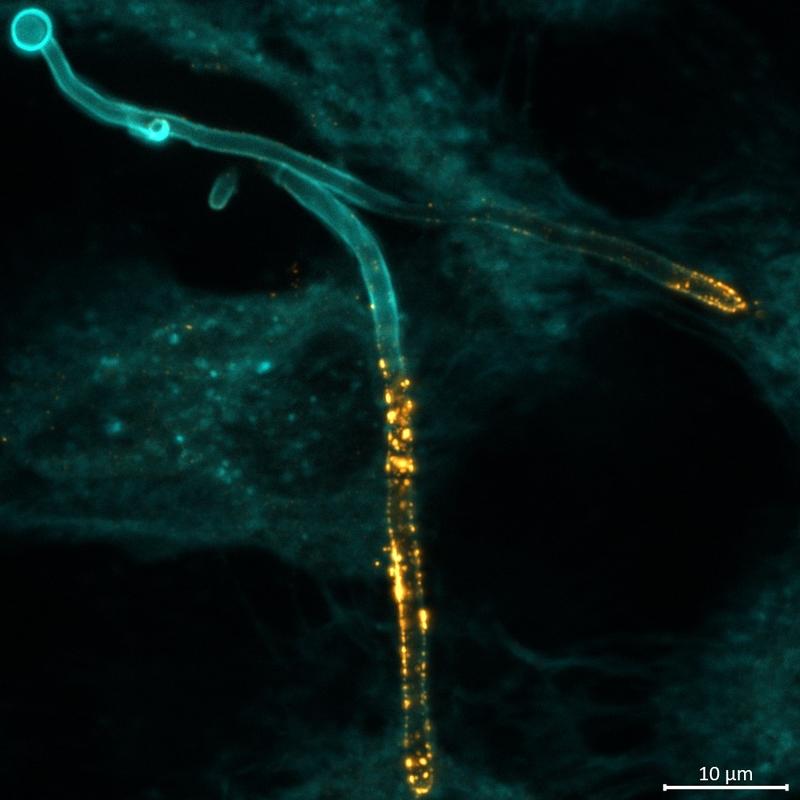
In further experiments, the researchers were able to show that the toxin has an extreme tendency to self-aggregate and clump together. The researchers then altered the Ece1 structure, which disrupted the correct folding of the protein. In laboratory experiments, this manipulation inhibited the formation of Candida albicans fungal hyphae, which also impaired the release of Candidalysin: the toxin clumped together in the cells and became ineffective. “Our results indicate that every single peptide in the Ece1 precursor protein is necessary for the release of Candidalysin. The main function of the precursor protein is apparently to prevent the toxin from clumping together,” explains first author Rita Müller. “This allows the fungal cells, the hyphae, to function normally, while the toxin can easily reach the host cells and cause damage there,” adds the second first author Annika König.
Fighting the toxin with nanobodies
In another study published in the journal mBio, the researchers focused on a new strategy for neutralizing Candidalysin: the use of nanobodies.
Nanobodies are synthetically produced antibodies. They were originally obtained from the blood of camels or llamas. They are much smaller and have a simpler structure than their human counterparts, which makes them much more flexible than human antibodies. Nanobodies are able to specifically dock onto the binding sites of the pathogens with which they attach to the host cells. In this way, they block the pathway for the pathogens and prevent infection. This principle has now been used for the first time to render Candidalysin harmless.
In vaginal infections with Candida albicans, Candidalysin causes damage to the mucosal tissue and thus promotes inflammatory overreactions of the immune system, which contribute to the worsening of symptoms. Treatment options are currently very limited, particularly for the chronic, recurring form of vaginal candidiasis. Yet this disease affects around 138 million women worldwide every year. The researchers therefore investigated the effect of nanobodies on the fungal infection. Laboratory tests have shown that special nanobodies can actually block the activity of Candidalysin. In the laboratory, the nanobodies very effectively reduced toxin-induced tissue damage, which would also inhibit inflammatory secondary reactions. The nanobodies were localized by the researchers using a special microscopy method – immunofluorescence – directly at the tissue sites colonized by the fungal hyphae. With the help of further modelling in the laboratory and on the computer, it was also possible to predict the dosage and timing of the nanobody addition in order to neutralize the toxin.
Nanobodies directed against Candidalysin could therefore be helpful in the treatment of complicated recurrent cases of vaginal candidiasis. A comparison with the frequently used antifungal drug fluconazole showed that the combination of both forms of therapy is particularly effective. “Together with this antifungal drug, the use of nanobodies could therefore represent a real treatment option for the disease,” emphasizes first author Marisa Valentine. “By neutralizing Candidalysin, the hyperinflammatory symptoms are alleviated. This could be of great importance for many patients,” says her colleague Paul Rudolph, also first author of the study.
The above-mentioned scientists are working on Candidalysin and its influence on the infection process under the supervision of Bernhard Hube and Marc Thilo Figge as part of their dissertations. Hube is head of the Department of Microbial Pathogenicity Mechanisms, Figge is group leader for Applied Systems Biology at the Leibniz-HKI. Both are professors at the Friedrich Schiller University Jena. The work was funded as part of the Cluster of Excellence Balance of the Microverse by the German Research Foundation (DFG), the Free State of Thuringia, the Federal Ministry of Education and Research (BMBF) and the European Union, and was also supported by the Wellcome Trust, the National Institutes of Health (NIH) and the EU’s Horizon 2020 network FunHoMic.
Wissenschaftlicher Ansprechpartner:
For "Secretion of the fungal toxin candidalysin is dependent on conserved precursor peptide sequences" in "Nature Microbiology":
- Bernhard Hube
Microbial Pathogenicity Mechanisms
+49 3641 532-1401
bernhard.hube@leibniz-hki.de
For "Nanobody-mediated neutralization of candidalysin prevents epithelial damage and inflammatory responses that drive vulvovaginal candidiasis pathogenesis" in "mBio":
- Bernhard Hube
Microbial Pathogenicity Mechanisms
+49 3641 532-1401
bernhard.hube@leibniz-hki.de
- Mark Gresnigt
Adaptive Pathogenicity Strategies
+49 3641 532-1305
mark.gresnigt@leibniz-hki.de
Originalpublikation:
Müller, R., König, A., Groth, S. et al. Secretion of the fungal toxin candidalysin is dependent on conserved precursor peptide sequences. Nat Microbiol 9, 669–683 (2024). https://doi.org/10.1038/s41564-024-01606-z
Valentine M, Rudolph P, Dietschmann A, Tsavou A, Mogavero S, Lee S, Priest EL, Zhurgenbayeva G, Jablonowski N, Timme S, Eggeling C, Allert S, Dolk E, Naglik JR, Figge MT, Gresnigt MS, Hube B. 2024. Nanobody-mediated neutralization of candidalysin prevents epithelial damage and inflammatory responses that drive vulvovaginal candidiasis pathogenesis. mBio 15:e03409-23.
https://doi.org/10.1128/mbio.03409-23
Ähnliche Pressemitteilungen im idw