Mechanism of action of the hepatitis B and D virus cell entry inhibitor bulevirtide deciphered
Over 12 million people worldwide are chronically infected with the hepatitis D virus (HDV). HDV uses the surface proteins of the hepatitis B virus (HBV) as a vehicle to specifically enter liver cells via a transporter protein in the cellular membrane. This cell entry can be prevented by the active agent bulevirtide, which is approved as a drug under the name Hepcludex. An international research team has now succeeded in deciphering the structure of bulevirtide in complex with the HBV/HDV receptor NTCP at the molecular level.
Chronic infection with the hepatitis D virus—the most serious viral liver disease—is associated with a high risk of dying from liver cirrhosis and liver cancer. The hepatitis D virus (HDV) uses the surface proteins of the hepatitis B virus (HBV) as a vehicle to specifically enter liver cells via a protein in the cellular membrane—the bile salt transporter protein NTCP. This cell entry can be prevented by the active agent bulevirtide. An international research team has now succeeded in deciphering the molecular structure of bulevirtide in complex with the HBV/HDV receptor NTCP at the molecular level. The research results published in the renowned journal Nature Communications pave the way for more targeted and effective treatments for millions of people chronically infected with HBV/HDV.
The entry inhibitor bulevirtide is the first and currently only approved drug (under the drug name Hepcludex) for the treatment of chronic infections with the hepatitis D virus. The active agent effectively inhibits the replication of hepatitis D viruses and leads to a significant improvement in liver function. However, the exact mechanism by which bulevirtide interacts with the virus entry receptor on the surface of the liver cells—the bile salt transporter protein NTCP (short for: sodium taurocholate cotransporting polypeptide)—and thereby inhibits the entry of the viruses into the cells was previously unknown.
In order to understand the molecular interaction of bulevirtide and NTCP at the molecular level, the researchers first generated an antibody fragment that specifically recognises the NTCP-bulevirtide complex and makes it accessible for analysis when bound to nanoparticles. This complex was then analysed using cryo-electron microscopy, which allowed to visualise structural details with atomic resolution. The research results represent a milestone in understanding both the interaction of HBV and HDV with their cellular entry receptor NTCP and the mechanism of cell receptor blockade by bulevirtide.
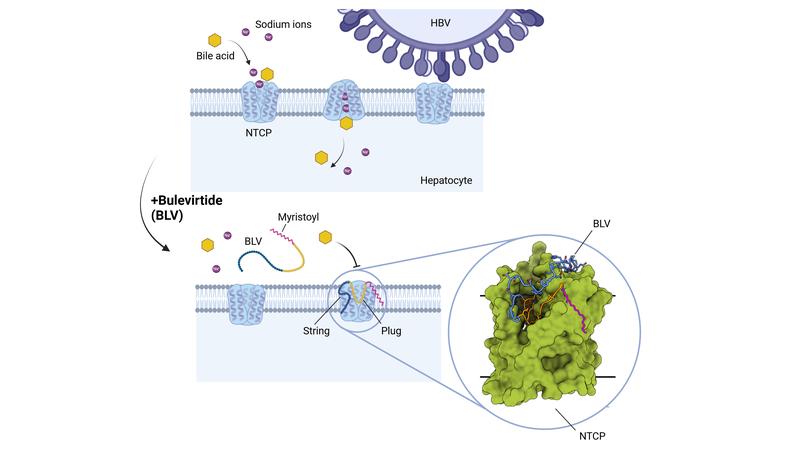
How bulevirtide blocks the cell entry receptor NTCP
The analysis showed that bulevirtide forms three functional domains in the interaction with the HBV/HDV receptor NTCP: a myristoyl group that interacts with the cell membrane on the outside of the cell; an essential core sequence ('plug') that fits precisely into the bile salt transport tunnel of the NTCP like the bit of a key into a lock; and an amino acid chain that stretches across the extracellular surface of the receptor, enclosing it like a brace.
"The formation of a 'plug' in the transport tunnel and the associated inactivation of the bile salt transporter is so far unique among all known virus-receptor complexes. This structure explains why the physiological function of the NTCP is inhibited when patients are treated with bulevirtide," says Prof Stephan Urban, DZIF Professor of Translational Virology and Deputy Coordinator of the DZIF research area Hepatitis, in whose laboratory at Heidelberg University the active agent bulevirtide was developed.
"Thanks to the structural details of the interaction with bulevirtide, we have also gained insights that enable the development of smaller active agents—so-called peptidomimetics—with improved pharmacological properties. Our structural analysis also lays the foundation for the development of drugs that are not only based on peptides and possibly enable oral administration," adds the co-author of the study, Prof Joachim Geyer from the Institute of Pharmacology and Toxicology at Justus Liebig University Giessen.
Evolutionary adaptation of hepatitis B viruses to host species
The structural analysis also helped to decode an important factor in the species specificity of hepatitis B and D viruses. According to the findings of the analysis, the amino acid at position 158 of the NTCP amino acid chain plays an essential role in virus-receptor interaction. A change in the amino acid at this position prevents the binding of HBV/HDV. This explains why certain Old World monkeys, such as macaques, cannot be infected by HBV/HDV.
"Our findings enable a deeper understanding of the evolutionary adaptation of human and animal hepatitis B viruses to their hosts and also provide an important molecular basis for the development of new and targeted drugs," adds co-author Prof Dieter Glebe, DZIF scientist at the Institute of Medical Virology at Justus Liebig University Giessen.
"Our research results are an important step in the fight against hepatitis D and B. By understanding the structure of bulevirtide and its binding to NTCP, we can potentially develop more targeted and effective treatments for millions of people chronically infected with HBV/HDV," says Prof. Kaspar Locher, last author of the publication and head of the internationally renowned structural biology team at ETH Zurich, summarising the study results.
Wissenschaftlicher Ansprechpartner:
Dr Dieter Glebe
Justus Liebig University Giessen
dieter.glebe@viro.med.uni-giessen.de
Dr Stephan Urban
Heidelberg University
dzifheidelberg.HYG@med.uni-heidelberg.de
Originalpublikation:
Liu H. et al.: Structure of antiviral drug bulevirtide bound to hepatitis B and D virus receptor protein NTCP, Nat Commun, 2024, DOI: https://doi.org/10.1038/s41467-024-46706-w
Weitere Informationen:
https://www.dzif.de/en/mechanism-action-hepatitis-b-and-d-virus-cell-entry-inhibitor-bulevirtide-deciphered Press release of the German Center for Infection Research (DZIF)
Ähnliche Pressemitteilungen im idw