New analgesic could replace opioids over the long term
Researchers at Mainz University have identified a natural active substance that may prove to be an effective alternative to opioids in the long run and could also help mitigate the opioid crisis
Opioids have long been known as natural substances with substantial pharmacological effect. They have been used as effective painkillers. A very prominent example is morphine, which was first isolated and synthesized in the early 19th century. It is a relief for severely ill patients in the last phases of their lives. However, when opioids are used inappropriately they can cause addiction and even the development of extremely serious undesirable effects, such as respiratory depression. In the USA, opioids were once widely promoted through the media and, as a consequence, were often prescribed to treat what were in fact mild disorders. According to the Centers for Disease Control and Prevention (CDC), there were nearly 645,000 cases of mortality due to opioid overdose in the United States between 1999 to 2021. And the opioid crisis has arrived in Germany, too. The main problem is street drugs and the fact that the synthetic opioid heroin, in particular, is cut with other, cheaper opioids, such as fentanyl. While a dose of 200 milligrams heroin is fatal, just two milligrams of fentanyl can kill. In 2022, more than 1,000 people in Germany died as a result of the consumption of opioids.
Starting with a database of 40,000 natural substances
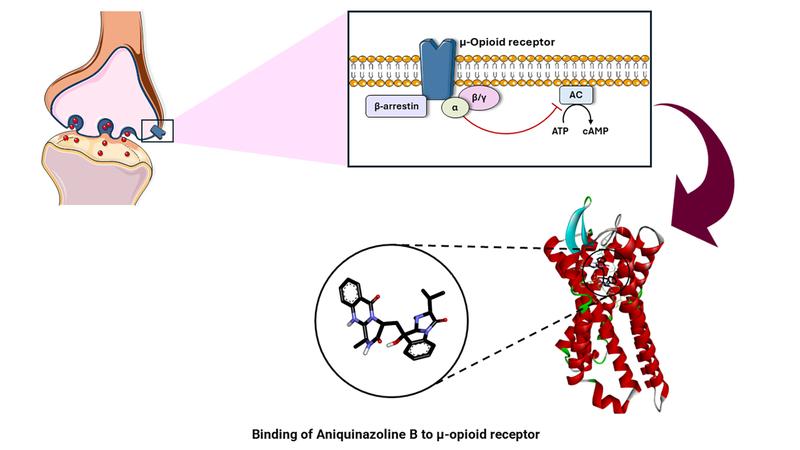
Governments have introduced measures to contain this epidemic. However, opioid addiction rates are high. Others suffer from extreme pain that needs to be alleviated. There is thus an urgent need for safe analgesics. Researchers at Johannes Gutenberg University Mainz (JGU) – with financial support of the Research Training Group "Life Sciences – Life Writing", funded by the German Research Foundation (DFG) – have now made progress towards this goal. "A natural product called aniquinazolin B that is isolated from the marine fungus Aspergillus nidulans stimulates the opioid receptors and could possibly thus be used instead of opioids in future," explained Roxana Damiescu, a member of the research team headed by Professor Thomas Efferth.
In the search for new compounds, the team started with a chemical database of more than 40,000 natural substances. It was their aim to determine how effectively each substance would bind to the corresponding receptor. And, in addition, they had to ascertain whether these had the properties required of a pharmaceutical drug. Such a compound must be water-soluble to some extent, for example. This research required calculations in the form of approximations, with the results becoming increasingly more precise the more frequently these calculations were performed. Each substance was the subject of some 750,000 individual calculations. Such a colossal number of calculations would vastly exceed the capacity of a standard PC. Therefore, the team utilized the MOGON supercomputer at JGU. The top 100 candidate products of these calculations were subsequently assessed using other analytical methods.
Further detailed investigation in the lab
The resultant top ten found their way into the lab, where they underwent biochemical analysis. The initial priority was to establish safety. Using preparations of human kidney cells, the researchers looked at whether higher concentrations of each substance would prove toxic to the cells and even kill them. Finally, two other aspects had to be subjected to testing. "We needed to confirm that the high binding energy of the substances to the pain receptors that had been predicted by the theoretical calculations was actually also produced in the real physical world," said Professor Thomas Efferth, head of the JGU Department of Pharmaceutical Biology. However, binding of a substance to the receptors is not alone sufficient. The binding must also influence the functioning of the receptors. Thus, the research team used a second test system to assess whether there was the kind of inhibition of biological activity that occurs on opioid use. One of the two compounds passed all tests with flying colors: aniquinazolin B, the substance present in the marine fungus Aspergillus nidulans. "The results of our investigations indicate that this substance may have effects similar to those of opioids. At the same time, it causes far fewer undesirable reactions," concluded Roxana Damiescu.
The corresponding research paper has recently been published in ChemMedChem.
Image:
https://download.uni-mainz.de/presse/09_pharma_pharmazeutische_biologie_opioide.jpg
Presentation of the binding of Aniquinazoline B to an opioid receptor
ill./©: Mohamed Elbadawi / JGU
Related links:
• https://ak-efferth.pharmazie.uni-mainz.de – Department of Pharmaceutical Biology at the JGU Institute of Pharmaceutical and Biomedical Sciences
• https://www.grk.lifesciences-lifewriting.uni-mainz.de/ – DFG-funded Research Training Group "Life Sciences – Life Writing" at JGU
• https://hpc-en.uni-mainz.de/ – High Performance Computing at JGU
Read more:
• https://press.uni-mainz.de/researchers-identify-potential-active-substances-against-coronavirus-by-running-supercomputer-simulations/ – press release "Researchers identify potential active substances against coronavirus by running supercomputer simulations" (5 May 2020)
Wissenschaftlicher Ansprechpartner:
Professor Dr. Thomas Efferth
Department of Pharmaceutical Biology
Institute of Pharmaceutical and Biomedical Sciences
Johannes Gutenberg University Mainz
55099 Mainz, GERMANY
phone: +49 6131 39-25751
e-mail: efferth@uni-mainz.de
https://ak-efferth.pharmazie.uni-mainz.de/people/head-of-department/
Originalpublikation:
R. Damiescu et al., Aniquinazoline B, a fungal natural product, activates the µ-opioid receptor
ChemMedChem, 23 May 2024,
DOI: 10.1002/cmdc.202400213
https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/cmdc.202400213
Die semantisch ähnlichsten Pressemitteilungen im idw