Sustainably produced covalent organic frameworks for efficient CO2 capture
Chemistry: Publication in Nature Communications
An international research team headed by Heinrich Heine University Düsseldorf (HHU) and the University of Siegen has synthesised a new compound, which forms a so-called covalent organic framework. The compound, which is based on condensed phosphonic acids, is stable and can for example be used to capture carbon dioxide (CO2), as the researchers describe in the scientific journal Nature Communications.
Covalent organic frameworks (for short: COFs) are a class of porous crystalline materials, which form scaffold-like structures. The term “covalent” denotes that chemical bonds between the individual building blocks of the framework are formed via shared electron pairs.
A research team headed by Dr Gündoğ Yücesan, Heisenberg Junior Research Group Leader at the Section for Nanoporous and Nanoscale Materials at HHU and Professor Dr Jörn Schmedt auf der Günne, leader of the Inorganic Materials Chemistry group at the University of Siegen, now presents a simple approach to this family of frameworks, the members of which are particularly stable and promise great application potential. Researchers from Berlin, Bremen, Saarbrucken, Turkey and the United Kingdom were also involved in the study, which has now been published in Nature Communications.
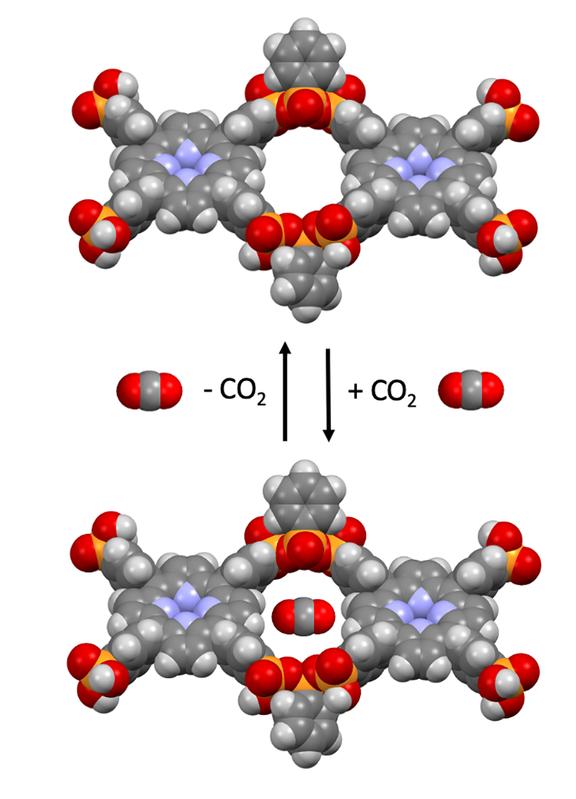
The class of polyphosphonate covalent organic frameworks is characterised by phosphorus-oxygen-phosphorus bonds, which comprise simple organic phosphonic acid building blocks and – almost like Lego bricks – can be joined together by heating them to temperatures of just approx. 200 degrees Celsius.
Dr Yücesan: “The special property of these COFs is that, despite the mild synthesis conditions, they exhibit good water and water vapour stability, meaning that – by contrast with compounds developed to date – they can be used in water and electrolytes.”
A further milestone was the development of a sustainable synthesis route. Yücesan: “For the first time, a solid-state synthesis process has been developed for COFs, which can be realised completely without solvents. This method enables low-cost, scalable production from kilogrammes to tonnes, making it more cost-effective compared with other microporous materials.
One challenge for the researchers was that the compounds did not crystallise well and are amorphous. They succeeded in finding evidence for the bonds by means of nuclear magnetic resonance. Professor Schmedt auf der Günne: “If we had not been able to use the common states of neighbouring phosphorus atom nuclei, the bonding structure of the substance would have remained in the dark and the properties would not have been understood.”
Polyphosphonates of this type have great application potential. The framework structures can capture the harmful greenhouse gas CO2. A slight change in pressure can release it again. “Such substances are needed for waste gas cleaning and to prevent greenhouse gas emissions,” the authors of the study note.
Originalpublikation:
Ke Xu, Robert Oestreich, Takin Haj Hassani Sohi, Mailis Lounasvuori, Jean G. A. Ruthes, Yunus Zorlu, Julia Michalski, Philipp Seiffert, Till Strothmann, Patrik Tholen, A. Ozgur Yazaydin, Markus Suta, Volker Presser, Tristan Petit, Christoph Janiak, Jens Beckmann, Jörn Schmedt auf der Günne & Gündoğ Yücesan. Polyphosphonate covalent organic frameworks. Nature Communications 15, 7862 (2024).
DOI: 10.1038/s41467-024-51950-1
Ähnliche Pressemitteilungen im idw