3D structures of biomolecules – “dictionaries” make fluorescence-based data accessible
Physical Chemistry: Publication in Nature Methods
A research team from Germany and the USA led by Heinrich Heine University Düsseldorf (HHU) has developed a data description that can provide results from fluorescence measurements for structural and dynamic modelling of large biomolecules. The authors explain in the scientific journal Nature Methods that, for the first time, other researchers can access fluorescence-based integrative structural models and their dynamics through databases. This provides experiment-based training data for the next generation of AI tools for dynamic structure modelling.
Proteins and nucleic acids are the central building blocks of life in all organisms. These biomolecules are made up of many individual building blocks, such as amino acids in the case of proteins. When the individual building blocks are assembled in the cells, the biomolecules are formed as complex, three-dimensional structures. Their specific shape is determined by the configuration and forces between the building blocks. However, in order to understand the function of biomolecules, it is important to consider not only their three-dimensional structure but also the number of different structural states and the exchange dynamics between them.
For a long time, it was very complex and time-consuming to determine the 3D structure of biomolecules using classical biophysical methods. In order to simplify and systematise this work step by step, all these three-dimensional structures have been collected in the “Protein Data Bank” (PDB) [Link: https://www.rcsb.org] since 1971. These more than 220,000 structures are used by AI-based tools such as “AlphaFold” - for whose development the Nobel Prize in Chemistry was awarded this year - as training data for neural networks. AI systems are already making good predictions of biomolecular structures. However, the tools are currently unable to predict dynamics due to a lack of experimental data.
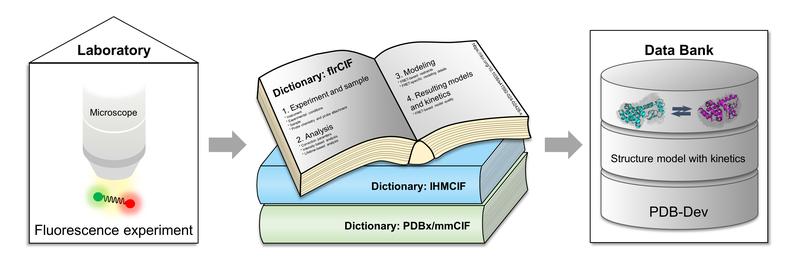
It is therefore important to use powerful experimental methods, such as fluorescence spectroscopy, which provide combined information about the dynamics and structure of complex biomolecules. In fluorescence experiments, specific interesting parts of the biomolecules are marked with small dye molecules that light up when excited externally and thus reveal their positions. Integrative modelling approaches combine such experimental data with computational methods to achieve higher structural resolution and take dynamics into account.
Dr Christian Hanke, postdoc at the HHU Institute of Molecular Physical Chemistry and first author of the article published in Nature Methods, emphasises: “Fluorescence experiments provide detailed information, making them an excellent data source for integrative modelling. However, to fully utilise this wealth of information, it must be made accessible to and usable by a broad scientific community.”
In the publication, the research team from HHU, Rutgers State University of New Jersey and the University of California in San Francisco presents a standardised data description in the form of three linked “dictionaries” that are organised in a common library. Prof Dr Claus Seidel from HHU, one of the two corresponding authors: “This organisational principle with combined dictionaries enables researchers for the first time to store integrative structural models based on fluorescence data together with kinetic information. At the same time, the general definitions can also be used by other methods to document dynamic properties of biomolecules alongside their structure in the database.”
This approach is necessary in order to link static structural models with the underlying energy landscape - i.e. the energy differences between different three-dimensional arrangements of the building blocks within the biomolecule. Prof Seidel: “This information makes it possible to develop and train the next generation of AI-based programs for predicting dynamic biomolecular structures. Here, the data obtained from fluorescence experiments on functionally relevant dynamics can make a very important contribution.”
This work was carried out as part of Prof Seidel's ERC Advanced Grant “hybridFRET”.
Originalpublikation:
Christian A. Hanke, John D. Westbrook, Benjamin M. Webb, Thomas-O. Peulen, Catherine L. Lawson, Andrej Sali, Helen M. Berman, Claus A. M. Seidel & Brinda Vallat. Making fluorescence-based integrative structures and associated kinetic information accessible. Nature Methods (2024).
DOI: 10.1038/s41592-024-02428-x
Die semantisch ähnlichsten Pressemitteilungen im idw