New Insights into the Mechanisms of Potassium Channels for Medicine
Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP) in Berlin, the Faculty of Medicine at Kiel University, and the Leibniz Institute of Virology in Hamburg are jointly receiving funding of nearly one million euros as part of the "Leibniz Cooperative Excellence" program. The goal of the project is to unravel fundamental mechanisms of two-pore domain potassium channels (K2P channels) – with potential new therapeutic approaches for cancer, autoimmune diseases, infectious diseases, central nervous system disorders, and cardiovascular diseases.
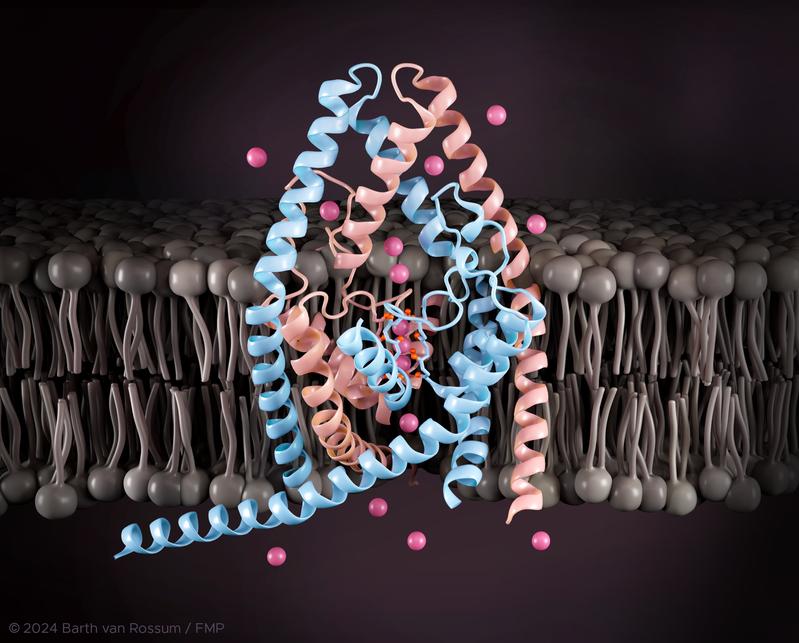
Two-pore domain potassium channels (K2P channels) are essential for maintaining the electrical balance and normal activity of various cell types. Disruptions in the functionality of K2P channels can lead to severe health problems such as atrial fibrillation, respiratory depression, pulmonary hypertension, sleep apnea, neuropathic pain, migraines, and depression. Consequently, these ion channels are promising targets for the development of new drugs. However, understanding how these channels function, particularly the mechanisms by which they respond to external stimuli and open or close – a process known as "gating" – remains a major challenge.
The research project "Deciphering the atomistic mechanism of selectivity filter gating in K2P channels," led by Prof. Han Sun, successfully secured funding of nearly one million euros through the Leibniz Competition 2025. The aim of the project is to gain a deeper understanding of the gating process in K2P channels. A particularly challenging aspect is studying the intrinsically disordered terminal regions of these channels, which play a crucial role in regulating gating. Furthermore, the project aims to develop new strategies for designing highly affine and highly selective small-molecule modulators targeting various K2P channels. Ion channels are among the most frequently targeted protein classes in drug development. The methods developed in this study could also be applied to designing drug-like molecules for other ion channels or viral proteins.
The project is being carried out by an interdisciplinary consortium with outstanding expertise in various methodologies, including cryo-electron microscopy (Prof. Maya Topf, Leibniz Institute for Virology), solid-state NMR (Prof. Adam Lange, FMP Berlin), molecular dynamics simulations (Prof. Han Sun, FMP Berlin), and electrophysiology (Dr. Marcus Schewe and Prof. Thomas Baukrowitz, Faculty of Medicine, Kiel University).
Project leader Prof. Han Sun investigates the functionally relevant dynamics of membrane proteins at the FMP, with a particular focus on ion channels. Her research group employs a variety of theoretical approaches, including molecular dynamics simulations, to explore the dynamics of proteins across different timescales. Additionally, the group develops innovative theoretical methods to predict active modulators with high affinity and specificity. Another central focus is integrating experimental data into simulations.
Prof. Adam Lange, also at the FMP in Berlin, uses solid-state NMR and other structural and biophysical techniques to study the structure and dynamics of membrane proteins in their natural environment, the cell membrane. The dynamics of membrane proteins often play a crucial role in their function. His group focuses on ion channels and intramembrane proteases. In the case of K2P channels, special attention is given to the intrinsically disordered terminal regions, which are essential for the regulation of these channels and can only be effectively studied using NMR spectroscopy. These planned studies will greatly benefit from the recently installed 1.2 GHz NMR spectrometer at the FMP, one of the most powerful devices of its kind worldwide.
The physiologists of Kiel University Dr. Marcus Schewe and Prof. Thomas Baukrowitz investigate structure-function relationships in various ion channels, with a current research focus on K2P channels. Their goal is to elucidate the molecular mechanisms regulating K2P channels through endogenous stimuli (e.g., lipids, pH, temperature, mechanical stress) and pharmacological drugs, primarily using molecular biological and electrophysiological techniques. These regulatory mechanisms are central to the understanding of the physiological and pathophysiological relevance of K2Ps.
At the Leibniz Institute for Virology (LIV) in Hamburg, Prof. Maya Topf develops integrative modeling methods to elucidate the structures of macromolecular machines, with a focus on cryo-EM structures. Her lab’s recent work includes TEMPy-ReFF, a method that refines models in cryo-EM maps and improves the interpretation of flexible regions. In this proposal, her team will investigate the structure and dynamics of K2P channels using cryo-EM, particularly the SF gating mechanism, and model ligand binding sites, relying on their expertise in small-molecule docking (ChemEM).
Wissenschaftlicher Ansprechpartner:
Prof. Dr. Han Sun
Structural Chemistry and Computational Biophysics
Leibniz-Forschungsinstitut für Molekulare Pharmakologie (FMP)
hsun@fmp-berlin.de
Phone.: + 49 30 94793 573
www.leibniz-fmp.de/sun
Originalpublikation:
Ähnliche Pressemitteilungen im idw